What started as a vertical integration solution for paper 50 years back is today an innovative, specialty chemicals company serving customers across diverse industries.
Formation of Kaolin
Kaolin is a form of clay. Clays are formed by the weathering of rocks. Weathering of rocks can be classified as physical or chemical process. In case of kaolin, the chemical reaction between water, carbon dioxide and feldspar (rock) gives rise to kaolin clay deposits.
Feldspar (Rock) + Water + Carbon Dioxide (Gas) -> Kaolin (Clay)
The kaolin deposits are classified as primary when the chemical reaction takes place in-situ. The deposits are classified as secondary when the reaction has already taken place to form kaolin; after which the kaolin is washed away and deposited elsewhere
Chemical composition and structure
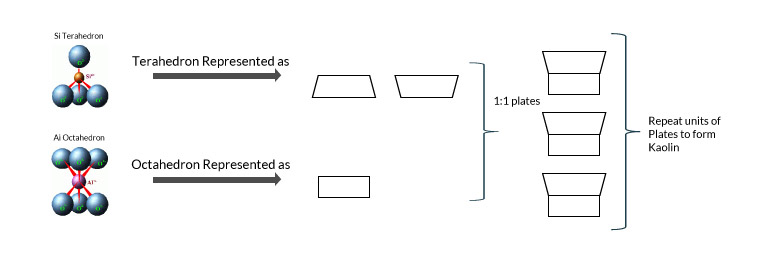
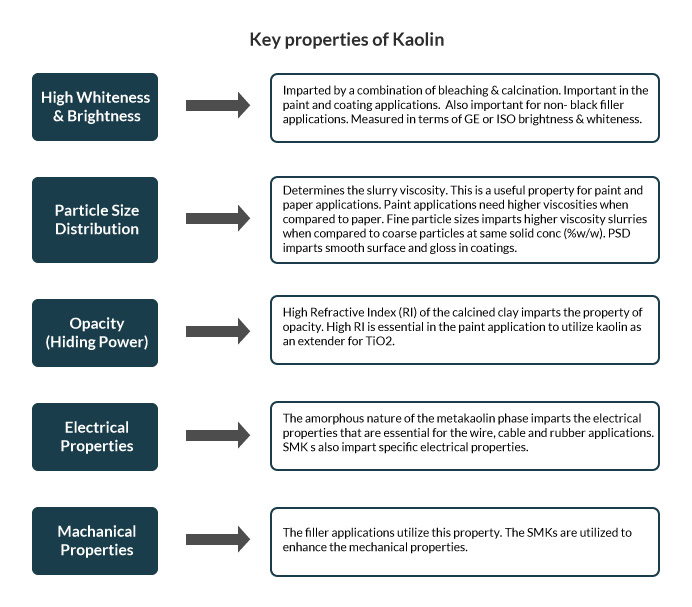
Chemically kaolin is identified by the formulae Al2Si2O5(OH)4, or as Al2O3·2SiO2·2H2O. Therefore Kaolin is an alumina-silicate as indicated by the formulae. Structurally kaolin is a platy mineral. Repeat units of Si-tetrahedron form the Si sheet; whereas repeat units of Al-octahedron form the Al-sheet. The tetrahedron and octahedron sheets from 1:1 plate formation to form kaolin.
Mining and Processing of Kaolin
In EICL facility the kaolin deposited are open-mined. The mined kaolin is termed as the matrix. The matrix can be classified on the basis of chemical and physical properties. The primary sorting of the matrix is done on the basis of colour (for example: white, sandy white, pink grey or yellow). The colour to the matrix is imparted by the various impurities (both inorganic and/or organic) present in the matrix.
Typically, presence of iron imparts a pink colour to the matrix. The mined and sorted kaolin is then subject to processing by a wet route to produce processed kaolins with desired properties for various applications. Matrix can be alternatively processed by a dry route; however the latter is only for certain applications. At EICL, all kaolins are processed via the wet route.
Difference between Hydrous and Calcined Kaolin
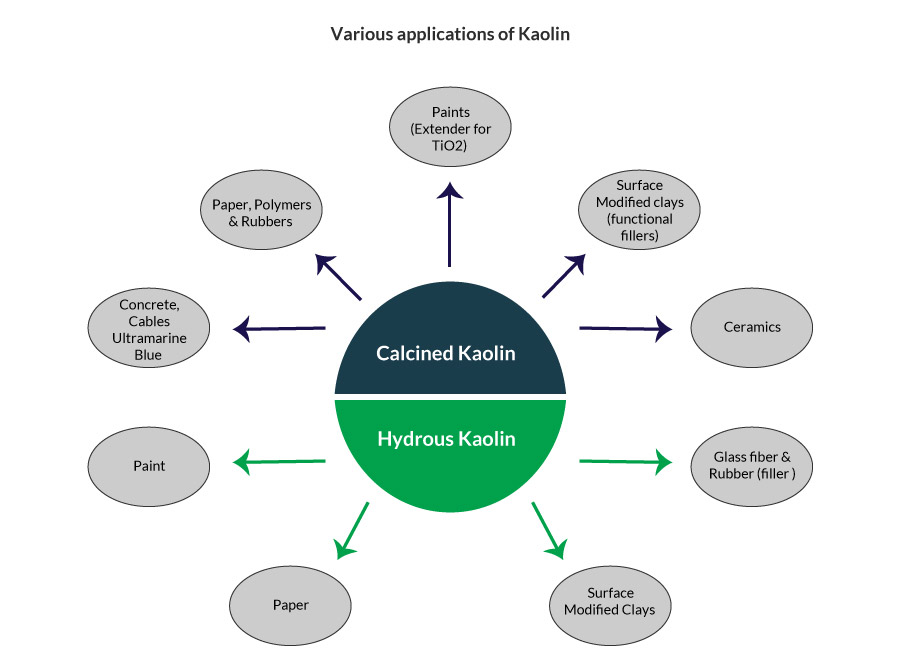
Kaolin is termed as hydrous when the kaolin has the bound water molecule. The presence of water imparts specific properties that are useful for certain industrial applications. They are generally used as fillers in the paper, paint, glass and rubber industries.
To lose the water molecule and improve colour, the hydrous clay is subjected to high temperatures and the process in known as calcination. The product is termed as calcined clay. The calcination in EICL is done in rotary calciners. By controlling the calcination temperature, Metakaolin (550–900°C), Spinel (925–1050°C) or Mullite Phases (>1050°C) of calcined kaolin can be produced.
Metakaolin has applications in cement, rubber, cables & ultramarine blue. The spinel phase is used mainly for paint applications as an extender for TiO2; it also has a few applications for paper, ceramics, polymer and rubber industries. The mullite phase finds its way into the ceramic industry.
Both hydrous and calcined kaolin are utilized for preparation of surface modified kaolin (SMK). The modification is aided by the use of silanes. The SMKs are utilized as functional fillers mainly for rubber and plastic applications to impart specific electrical and mechanical properties. SMKs can be utilized to substitute (fully/partially) the more expensive fillers such as carbon black in the tyre industry.
EICL facility for processing kaolin
EICL has kaolin mines and two wet processing units in Kerala. The calcined clay products are produced only from Thonakkal; whereas the rest is from both Veli & Thonakkal. The total installed capacity of EICL is 300,000 Tonnes of processed kaolin/annum. EICL has a wide range of products in the portfolio to cater to the various market needs. There is a sustainable focus for development of new products to provide value added service to the customer.