What started as a vertical integration solution for
paper 75 years back is today an innovative, specialty chemicals company serving customers across diverse industries.
Introduction to Starch
Starch is produced by all green plants from excess glucose produced during photosynthesis and serves the plant as a reserve food supply. The word “starch” is from a Germanic root with the meanings “strong, stiff, strengthen, stiffen”. Modern German Stärke (starch) is related. “Amylum” for starch is from the Greek αμυλον, “amylon” which means “not ground at a mill”. The root amyl is used in biochemistry for several compounds related to starch.
Starch is stored in chloroplasts in the form of granules and in such organs as the roots of the tapioca plant; the tuber of the potato; the stem pith of sago; and the seeds of corn, wheat, and rice. When required, starch is broken down, in the presence of certain enzymes and water, into its constituent monomer glucose units, which diffuse from the cell to nourish the plant tissues. In humans and other animals, starch is broken down into its constituent sugar molecules, which then supply energy to the tissues.
Starch grains from the rhizomes of Typha (cattails, bullrushes) as flour have been identified from grinding stones in Europe dating back to 30,000 years ago.[4] Starch grains from sorghum were found on grind stones in caves in Ngalue, Mozambique dating up to 100,000 years ago.
Pure extracted wheat starch paste was used in Ancient Egypt possibly to glue papyrus. The extraction of starch is first described in the Natural History of Pliny the Elder around AD 77-79. Romans used it also in cosmetic creams, to powder the hair and to thicken sauces. Persians and Indians used it to make dishes similar to gothumai wheat halva. Rice starch as surface treatment of paper has been used in paper production in China, from 700 AD onwards.
What is Starch?
Starch is a carbohydrate consisting of a large number of glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants as an energy store. It is the most common carbohydrate in human diets and is contained in large amounts in such staple foods as potatoes, wheat, maize (corn), rice, and cassava.
The white, granular, organic chemical is a soft, white, tasteless powder that is insoluble in cold water, alcohol, or other solvents. The basic chemical formula of the starch molecule is (C6H10O5)n.
Chemical Composition of Starch
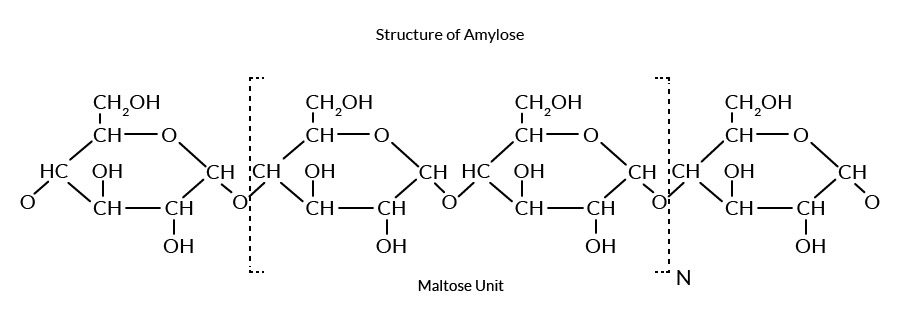
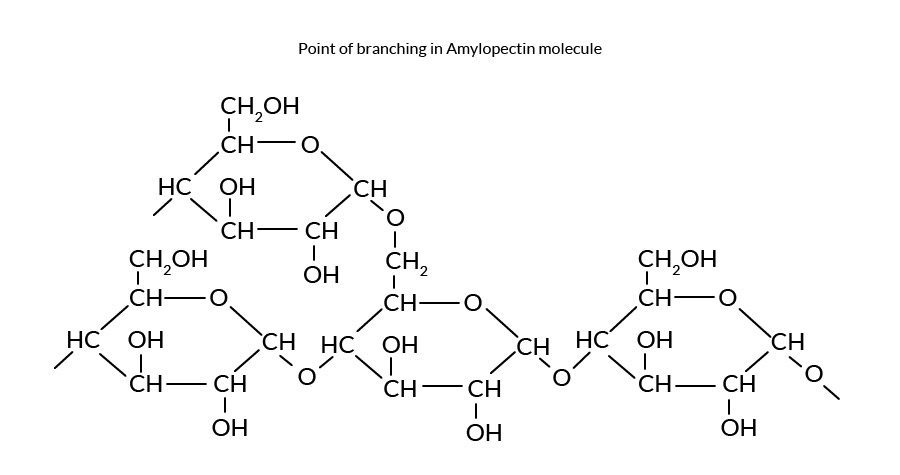
Starch is polymeric carbohydrate consisting of anhydroglucose(AG) units linked together 1- 4 & 1-6 glucosidic bonds , consisting of two major types of polymers. Starch can be separated into two fractions–amylose and amylopectin. Natural starches are mixtures of amylose (10-20%) and amylopectin (80-90%)
Sources of Starch
- Wheat
- Maize/Corn
- Potato
- Tapioca (Cassava)
- Rice
- Barley, Sorghum & other Cereal
Commercial Starch
Most commercial starch is made from corn, although wheat, tapioca, and potato starch are also used. Commercial starch is obtained by crushing or grinding starch-containing tubers or seeds and then mixing the pulp with water; the resulting paste is freed of its remaining impurities and then dried.
How is Starch Processed
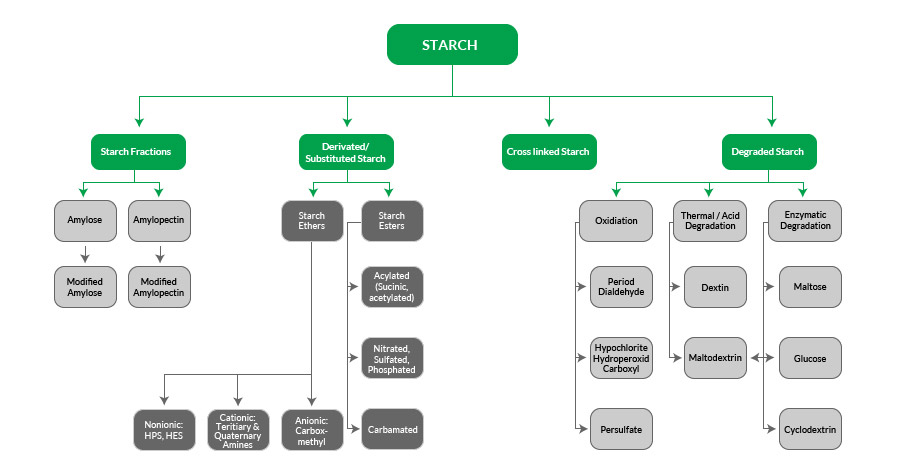
Starch Modification: Starch can be changed by chemical, physical and enzymatic treatments. Modifications give starch its specific and desired properties. Derivatisation technologies aim for modifications of the natural starch properties or for the establishment of new ones for utilisation of starch in different applications. Thus starch molecules are degraded, chemically modified by introduction of functional groups or changed by hydrothermal technology.
Starch becomes soluble in water when heated. The granules swell and burst, the semi crystalline structure is lost and the smaller amylose molecules start leaching out of the granule, forming a network that holds water and increasing the mixture’s viscosity. This process is called starch gelatinization. During cooking, the starch becomes a paste and increases further in viscosity. During cooling or prolonged storage of the paste, the semi-crystalline structure partially recovers and the starch paste thickens, expelling water. This is mainly caused by retrogradation of the amylose. This process is responsible for the hardening of bread or staling, and for the water layer on top of a starch gel.
If starch is subjected to dry heat, it breaks down to form dextrins, also called “pyrodextrins” in this context. This break down process is known as dextrinization. (Pyro)dextrins are mainly yellow to brown in color and dextrinization is partially responsible for the browning of toasted bread
Types of Starch
Key Properties of Starch
Viscosity, shear resistance, gelatinization, textures, solubility, tackiness, gel stability, cold swelling and retrogradation are all functions of their amylose/amylopectin ratio. When aiming at functional properties in starch, most commercial companies examine the characteristics of competitive starches in particular applications. Where the characteristics are unattainable then value additions are looked into. This could be as simple as sterilizing products required for the pharmaceutical industry to highly complex chemical modification to confer properties totally different from the native starch.
Granule size and structure: According to many sources, granule size does not, on its own, appear to have a strong effect on starch performance. It is, however, believed to be a contributing factor in how rapidly a starch gelatinizes and its gelatinization temperature. Large starch granules tend to build higher viscosity.
Amylose:amylopectin ratio: Waxy corn and common corn starches both have the same granule size, but waxy corn will swell to a greater degree and each will gelatinize at different temperatures. This is largely due to their differing amylose: amylopectin composition. Generally, the higher the amylose, the higher the gelatinization temperature.
Molecular structure of amylose and amylopectin: Longer amylose molecules tend to make a product’s texture stringy because of the way they associate. The molecular weight of the amylose also affects the elasticity of a gel.
Phosphorus: Starches contain phosphorus in some form or another. The nature of the phosphorus affects starch performance.
Various Applications of Starch
Besides the basic nutritional uses, starches are used in brewing and as thickening agents in baked goods and confections. The biggest industrial non-food use of starch is as adhesive in the papermaking process. Starch is used in paper manufacturing to increase the strength of paper and is also used in the surface sizing of paper. Starch is used in the manufacture of corrugated paperboard, paper bags and boxes, and gummed paper and tape. Large quantities of starch are also used in the textile industry as warp sizing, which imparts strength to the thread during weaving. Dissolving starch in warm water gives wheat paste, which can be used as a thickening, stiffening or gluing agent. Starch can be applied to parts of some garments before ironing, to stiffen them.
EICL Facilities for Processing Starch
EICL has its starch facilities at Yamunanagar, Haryana and Shimoga, Karnataka with an installed capacity of 80,000 metric tonnes/annum. The starch division at Yamunanagar can trace its origins back to 1937 when Mr Lala Karam Chand Thapar promoted a company by the name of Indian Starch & Chemicals Limited which later became Bharat Starch Industries limited. It consolidated its market dominance via the ‘Haathi’ brand and in 2001, Bharat Starch Industries became a part of English Indian Clays Limited.